How the West Controls Pharmaceutical Prices: Lessons for Malaysia
Malaysia Drug Price: Lessons from the West
Abstract
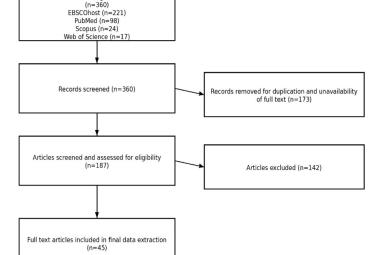
Drug price in Malaysia has increased substantially over the years. Malaysian policymakers may learn from the Western countries on how to control pharmaceutical spending. We conducted a review to assess the pharmaceutical strategies adopted in Western countries to curb pharmaceutical pricing and compared it with the current Malaysian system. The study found that the European countries adopted a regulated pharmaceutical market. In this regulated market, the price of the drugs will be determined using external reference pricing. The reimbursement and pricing of the drugs are also based on a set of guidelines rather than arbitrary nature. Additionally, the health technology assessment in the European countries utilised rating systems to categorise the added therapeutic benefit of the drugs. This rating system will add more information for price negotiation between the government and pharmaceutical manufacturers. Furthermore, cost-effectiveness analysis is also being incorporated into the decision-making process to ascertain the optimal use of scarce health resources. On top of that, pooled procurement system has been established in order to benefit from the higher volume purchasing. These measures may help Malaysia to ensure that the pharmaceutical spending remain sustainable and that Malaysians, will continue to have access toward a high-quality and affordable healthcare in the future.
Keywords :
drug industry,
HEALTH EQUITY,
Health policy,
health services,
pharmaceutical policy,
Abstrak
Harga ubatan di Malaysia telah naik mendadak sejak beberapa tahun lalu. Pembuat polisi di Malaysia boleh belajar dari negara-negara Barat tentang cara untuk mengawal kenaikan perbelanjaan farmaseutikal. Kami melakukan satu kajian untuk menilai strategi yang dilaksanakan di negara-negara Barat bagi mengawal harga ubatan dan membandingkannya dengan strategi di Malaysia. Kajian ini mendapati bahawa negara-negara Eropah mengamalkan pasaran terkawal bagi industri farmaseutikal. Melalui pasaran terkawal ini, harga ubatan ditentukan oleh harga rujukan luar. Harga ubatan ditentukan berdasarkan beberapa panduan berbanding penilaian secara rawak. Selain itu, penilaian teknologi kesihatan di Eropah menggunakan sistem penilaian bagi mengkategorikan manfaat tambahan ubat-ubatan. Sistem penilaian ini akan memberi lebih maklumat dalam perundingan antara kerajaan dan syarikat farmaseutikal. Tambahan pula, kajian keberkesanan kos diambil kira dalam membuat keputusan bagi memastikan sumber kesihatan yang terhad digunakan secara optimum. Sistem perolehan terkumpul digunakan untuk memanfaatkan harga yang lebih murah dari jumlah yang banyak. Langkah-langkah ini dapat membantu Malaysia memastikan perbelanjaan farmaseutikal kekal lestari dan rakyat Malaysia dapat terus memperoleh sistem kesihatan yang berkualiti tinggi dan berpatutan pada masa akan datang.
Kata Kunci :
harga ubatan,
liputan kesihatan sejagat,
polisi kesihatan,
Correspondance Address
Dr. Siew Chin Ong. School of Pharmaceutical Sciences, Universiti Sains Malaysia 11800 Gelugor, Penang, Malaysia. Tel: +604-653 3888 Email: siewchinong@usm.my, oschin99@yahoo.com